- Risk Group 1 (no or low individual and community risk) A microorganism that is unlikely to cause human or animal disease.
- Risk Group 2 (moderate individual risk, low community risk): A pathogen that can cause human or animal disease but is unlikely to be a serious hazard to laboratory workers, the community, livestock or the environment. Laboratory exposures may cause serious infection, but effective treatment and preventive measures are available and the risk of spread of infection is limited.
- Risk Group 3 (high individual risk, low community risk) A pathogen that usually causes serious human or animal disease but does not ordinarily spread from one infected individual to another. Effective treatment and preventive measures are available.
- Risk Group 4 (high individual and community risk): A pathogen that usually causes serious human or animal disease and that can be readily transmitted from one individual to another, directly or indirectly. Effective treatment and preventive measures are not usually available.
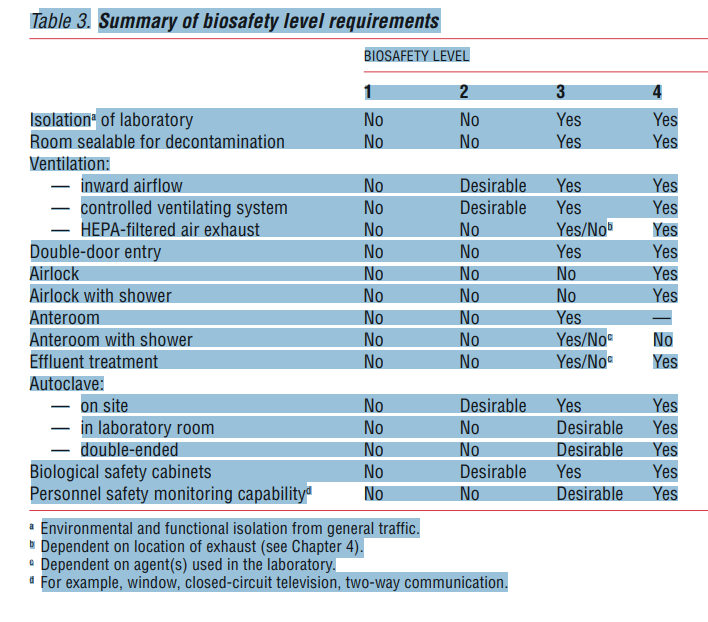
Laboratory facilities are designated as
- Basic – Bio-safety Level 1,
- Basic – Bio-safety Level 2,
- Containment – Bio-safety Level 3, and
- Maximum containment – Bio-safety Level 4.
Bio-safety level designations are based on a composite of the design features, construction, containment facilities, equipment, practices and operational procedures required for working with agents from the various risk groups.
Table 2 relates but does not “equate” risk groups to the bio-safety level of laboratories designed to work with organisms in each risk group. Countries (regions) should draw up a national (regional) classification of microorganisms, by risk group, taking into account:
- Pathogenicity of the organism.
- Mode of transmission and host range of the organism. These may be influenced by existing levels of immunity in the local population, density and movement of the host population, presence of appropriate vectors, and standards of environmental hygiene.
- Local availability of effective preventive measures. These may include:prophylaxis by immunization or administration of antisera (passive immunization); sanitary measures, e.g. food and water hygiene; control of animal reservoirs or arthropod vectors.
- Local availability of effective treatment. This includes passive immunization, post-exposure vaccination and use of antimicrobial, antivirals and chemotherapeutic agents, and should take into consideration the possibility of the emergence of drug-resistant strains.
The association of an agent with a bio-safety level for laboratory activity – must be based on a risk assessment. Such an assessment will take the risk group as well as extra factors into concern in determining the fitting bio-safety level. For example, an organism assigned to Risk Group 2 may normally require Bio-safety Level 2 facilities, equipment, practices and processes for safe bearing of work. However, if certain experiments involve the production of high-concentration aerosols, then Level 3 may be more appropriate to offer the needed degree of safety. Hence the bio-safety level assigned for the specific work to be carried out is determined by expert judgement based on a risk assessment, rather than by reflex assignment of a laboratory bio-safety level according to the particular risk group of the agent to be used
Non-Bio-hazardous Material
Biological materials that are not normally infectious are considered non-bio-hazardous.
This includes
- non-pathogenic microorganisms, viruses, and sub-viral agents;
- plants and non-primate animals (except those listed under bio-hazardous material above),
- biological material not likely to contain infectious agents and
- Biologically-derived, non-toxic molecules.