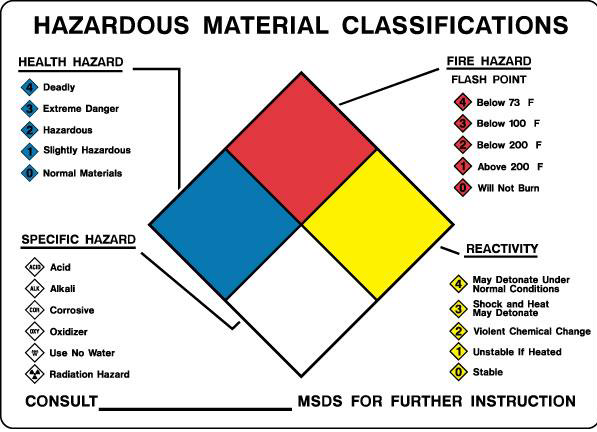
Hazardous chemicals possess a spectrum of physical, chemical, and toxicological properties that must be understood before beginning work in a laboratory. The following sections of this manual provide specific information for categories of common hazardous chemicals.
Explosive Chemicals
Explosive chemicals are chemicals that cause a sudden, almost instantaneous release of pressure, gas, and heat when subjected to shock, pressure, or high temperature. Because of the hazards associated with explosive chemicals, the campus prohibits the acquisition or purposeful production of explosive chemicals. Examples of explosive chemicals include
- ammonium nitrate
- ammonium perchlorate;
- barium azide;
- diazodinitrophenol;
- diethylene glycol dinitrate;
- dinitrophenolates;
- 2, 4 dinitrophenyl hydrazine;
- lead styphnate; nitrourea;
- nitrocellulose;
- sodium picramate;
- tetranitroanaline; and
- trinitrophenol (dry picric acid).
In addition to inherently explosive chemicals, some laboratory chemicals become potentially explosive if managed incorrectly. These chemicals, under certain conditions (gentle heat, light, mild shock, or chemical reaction) have the potential to undergo explosive reactions. For example, acetylides, azides, metal salts of nitrophenols, organic nitrates, multi-nitrated compounds, and organic peroxides become shock sensitive over time as they begin to dry or are mixed with metal oxides. Picric acid and picrylchloride become explosive if not sufficiently hydrated. To prevent laboratory chemicals from becoming explosive, consult the MSDS and adhere to the recommended storage and usage procedures outlined in both the MSDS and this manual. The Safety Officer should be contacted immediately if an explosive or potentially explosive chemical is discovered in the laboratory.